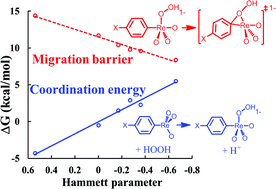
We studied the Baeyer–Villiger (BV) type oxidation of phenylrhenium trioxide (PTO) by H2O2 in the aqueous phase using Quantum Mechanics (density functional theory with the M06 functional) focusing on how the solution pH and the para-substituent affect the Gibbs free energy surfaces. For both PTO and MTO (methylrhenium trioxide) cases, we find that for pH > 1 the BV pathway having OH− as the leaving group is lower in energy than the one involving simultaneous protonation of hydroxide. We also find that during this organometallic BV oxidation, the migrating phenyl is a nucleophile so that substituting functional groups in the para-position of phenyl with increased electron-donating character lowers the migration barrier, just as in organic BV reactions. However, this substituent effect also pushes electron density to Re, impeding HOO− coordination and slowing down the reaction. This is in direct contrast to the organic analog, in which para-substitution has an insignificant influence on 1,2-addition of peracids. Due to the competition of the two opposing effects and the dependence of the resting state on pH and concentration, the reaction rate of the organometallic BV oxidation is surprisingly unaffected by para-substitution.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...