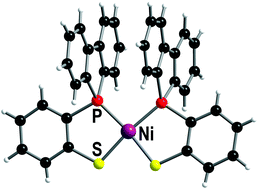
Reaction of the ditopic phosphanylarylthiol 1-P(Biph)-2-SHC6H4 (BiphPSH, Biph = 1,1′-biphenyl-2,2′-diyl), prepared by lithiation–electrophilic substitution, with NiCl2·6H2O, Na2[PdCl4] and [PtI2(cod)] (cod = 1,5-cyclooctadiene) in a 2 : 1 ratio and in the presence of NEt3 led to formation of exclusively cis isomers of the square-planar complexes cis-[M{(1-P(Biph)-2-S-C6H4)-κ2S,P}2] ([M{(BiphPS)-κ2S,P}2]; M = Ni (1), Pd (2), Pt (3)). Density functional calculations support the assumption that this is probably due to intramolecular π–π interaction of the biphenyl groups, which results in enhanced stability of the cis isomers. Compound 1 is the first example of a structurally characterised mononuclear cis-bis(phosphanylthiolato)nickel(II) complex. Small amounts of the trinuclear complex [{PtI(1-P(Biph)-μ-2-S-C6H4-κ2S,P)}3] (4) are also formed besides the mononuclear platinum bis-chelate complex 3.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...