Synthesis, purification, and characterization of phosphine oxides and their hydrogen peroxide adducts†
Abstract
Reactions of the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O·(H2O2)x (x = 0.5–1.0). Air
O·(H2O2)x (x = 0.5–1.0). Air ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and [Cy3P
O and [Cy3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O·(H2O2)]2 show a P
O·(H2O2)]2 show a P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stacking motif for the
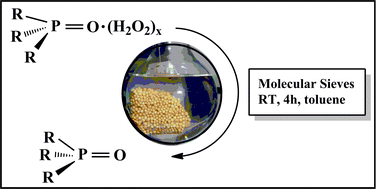
O stacking motif for the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O·(H2O2)x with molecular sieves destroys the bound H2O2 safely under mild conditions (room temperature,
O·(H2O2)x with molecular sieves destroys the bound H2O2 safely under mild conditions (room temperature,

 Please wait while we load your content...
Please wait while we load your content...