A potassium-promoted Mo carbide catalyst system for hydrocarbon synthesis
Abstract
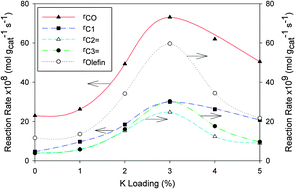
Potassium-promoted Mo carbide catalysts prepared by temperature-programmed carburization with H2–C3H8 have been evaluated for Fischer–Tropsch synthesis (FTS). Temperature-programmed carburization of MoO3–Al2O3 with a mixture of H2–C3H8 was a two-stage conversion involving the transformation of Mo oxide to the final carbide phase via an intermediate oxycarbide phase. Compensation effect and isokinetic relationship were observed for both oxycarbide and carbide phase formation suggesting that a similar topotactic mechanism was involved in the solid phase transformation. CO seemed to adsorb more strongly than H2 as implicated by its higher heat of desorption and site concentration. Both acid and basic centres were detected on the Mo carbide surface. The K-promoter increased site concentration for both weak and strong basic sites. The relationship between FT activity and K loading paralleled the trend in CO2 heat of desorption with K addition. FT activity attained a maximum at about 3%K loading. CO hydrogenation over the Mo carbide catalyst was most suitably described by an enolic intermediate mechanism. A generalized kinetic model was derived for predicting the reaction rate for individual hydrocarbons as a function of chain growth propagation and olefin-to-paraffin ratio.
- This article is part of the themed collection: Homogeneous and heterogeneous catalysis in industry

 Please wait while we load your content...
Please wait while we load your content...