Porous cross linked enzyme aggregates (p-CLEAs) of Saccharomyces cerevisiae invertase
Abstract
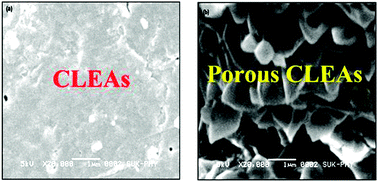
In this work, porous cross-linked enzyme aggregates (p-CLEAs) of Saccharomyces cerevisiae invertase are reported for the first time. Porous CLEAs were prepared by adding
- This article is part of the themed collection: Biocatalysis

 Please wait while we load your content...
Please wait while we load your content...