Cofactor promiscuity among F420-dependent reductases enables them to catalyse both oxidation and reduction of the same substrate
Abstract
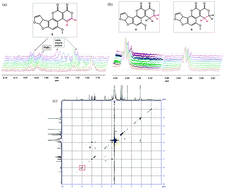
The recently reported F420H2-dependent reductases (FDRs) catalyse the reduction of
- This article is part of the themed collection: Biocatalysis

 Please wait while we load your content...
Please wait while we load your content...