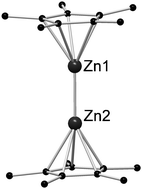
The discovery of decamethyldizincocene [Zn2(η5-Cp*)2] (Cp* = C5Me5), the first complex containing a covalent zinc–zinc bond, by Carmona in 2004 initiated the search for this remarkable class of compounds. Low-valent organozinc complexes can either be formed by ligand substitution reactions of [Zn2(η5-Cp*)2] or by reductive coupling reactions of Zn(II) compounds. To the best of our knowledge, until now 25 low-valent Zn–Zn bonded molecular compounds stabilized by a variety of sterically demanding, very often chelating, organic ligands have been synthesized and characterized. There are two major reaction pathways of [Zn2(η5-Cp*)2]: it can either react with cleavage of the Zn–Zn bond or by ligand substitution. In addition, upon reaction with late transition metal complexes, [Zn2(η5-Cp*)2] was found to form novel intermetallic complexes with Cp*Zn and Cp*Zn2 acting as unusual one-electron donor ligands. Very recently, the potential capability of [Zn2(η5-Cp*)2] to serve as a suitable catalyst in hydroamination reactions was demonstrated. Finally, the recent work on Cd–Cd bonded coordination compounds is reviewed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...