Density functional theory (DFT) was employed to study the water dissociation and water-gas shift (WGS) reaction on a series of inverse model catalysts, M3O3x/Cu(111) (M = Mg, Ti, Zr, Mo, W; x = 1, 2, 3). It has been found that the WGS reaction on Cu can be facilitated by introducing various oxides to lower the barrier of water dissociation. Accordingly, the calculated reaction energy for water dissociation was used as a scaling descriptor to screen the WGS activity of oxide–Cu model catalysts. Our calculations show that the activity towards water dissociation decreases in a sequence: Mg3O3/Cu(111) > Zr3O6/Cu(111) > Ti3O6/Cu(111) > W3O9/Cu(111), Mo3O9/Cu(111). It seems that Mg3O3/Cu(111) is the best WGS catalyst among the systems studied here, being able to dissociate water with no barrier. During the process, both Cu and oxides participate in the reaction directly. The strong M3O3x–Cu interaction is able to tune the electronic structure of M3O3x and therefore the activity towards water dissociation. Further studies of the overall WGS reaction on Mg3O3/Cu(111) show that water dissociation may not be the key step to control the WGS reaction on Mg3O3/Cu(111) and the removal of H from Mg3O3 can be problematic. The strong interaction between H and O from Mg3O3 blocks the O sites for further water dissociation and therefore the WGS reaction. Our study observes a very different behavior of oxide clusters in such small size from the bigger ones supported on Cu(111) and provides new insight into the rational design of the WGS catalysts.
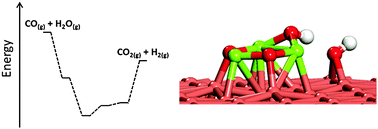
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
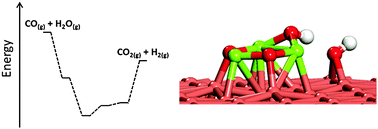

 Please wait while we load your content...
Please wait while we load your content...