The conversion of CO2 and CH4 to acetic acid over the Au-exchanged ZSM-5 catalyst: a density functional theory study†
Abstract
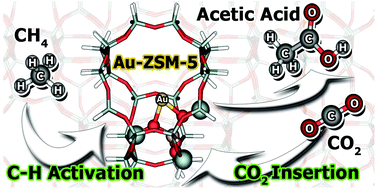
The direct conversion of methane and carbon dioxide to
- This article is part of the themed collection: Computational Catalysis and Materials for Energy Production, Storage and Utilization

 Please wait while we load your content...
Please wait while we load your content...