Optoelectronic properties of (ZnO)60 isomers†
Abstract
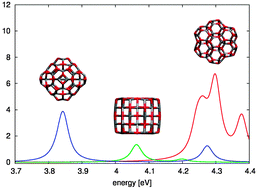
We studied the optoelectronic properties of six possible structures of the (ZnO)60 cluster using density functional theory (DFT). Vertical ionization energies and electron affinities are calculated through total energy differences, while the optical absorption spectra are obtained by using hybrid time-dependent DFT. The (ZnO)60 cluster has been proven to be particularly stable and it is of potential interest for future applications in nanoelectronics, but its ground-state configuration has been unknown to date. Since the relative stability inferred from total energy calculations suffers from a strong dependence on the computational scheme adopted, we combined it with optical spectroscopy to identify the most abundant geometrical structure of this cluster. The calculated optical spectra are different for each isomer and they could be thus used in comparison with experimental data to explain the ground state of (ZnO)60.

 Please wait while we load your content...
Please wait while we load your content...