Thermodynamics of water clusters under high pressures. A case study for (H2O)15 and (H2O)15CH4
Abstract
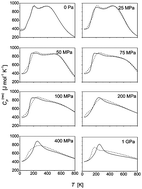
Thermal properties and structures of the water cluster containing fifteen molecules, either pure or doped with methane, are studied via classical parallel tempering Monte Carlo calculations in the isothermal–isobaric ensemble. The main emphasis is on structural transformations the cluster undergoes with increasing temperature and pressure. A simple TIP4P interaction model is employed for

 Please wait while we load your content...
Please wait while we load your content...