A computational investigation of ring-shift isomerization of sym-octahydrophenanthrene to sym-octahydroanthracene catalyzed by acidic zeolites†
Abstract
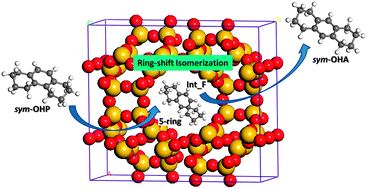
The ring-shift isomerization of sym-octahydrophenanthrene (sym-OHP) to sym-octahydroanthracene (sym-OHA) catalyzed by acidic
- This article is part of the themed collection: Computational Catalysis and Materials for Energy Production, Storage and Utilization

 Please wait while we load your content...
Please wait while we load your content...