Selected AB42−/− (A = C, Si, Ge; B = Al, Ga, In) ions: a battle between covalency and aromaticity, and prediction of square planar Si in SiIn42−/−†
Abstract
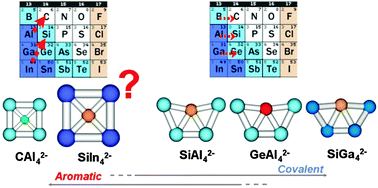
CAl42−/− (D4h, 1A1g) is a cluster ion that has been established to be planar, aromatic, and contain a tetracoordinate planar C atom. Valence isoelectronic substitution of C with Si and Ge in this cluster leads to a radical change of structure toward distorted pentagonal species. We find that this structural change goes together with the cluster acquiring partial covalency of bonding between Si/Ge and Al4, facilitated by hybridization of the atomic orbitals (AOs). Counter intuitively, for the AAl42−/− (A = C, Si, Ge) clusters, hybridization in the dopant atom is strengthened from C, to Si, and to Ge, even though typically AOs are more likely to hybridize if they are closer in energy (i.e. in earlier elements in the Periodic Table). The trend is explained by the better overlap of the hybrids of the heavier dopants with the orbitals of Al4. From the thus understood trend, it is inferred that covalency in such clusters can be switched off, by varying the relative sizes of the AOs of the main element and the dopant. Using this mechanism, we then successfully killed covalency in Si, and predicted a new aromatic cluster ion containing a tetracoordinate square planar Si, SiIn42−/−.
- This article is part of the themed collection: Predicting new molecules by quantum chemical methods

 Please wait while we load your content...
Please wait while we load your content...