Small carbides of third-row main group elements: structure and bonding in C3X compounds (X = K–Br)†
Abstract
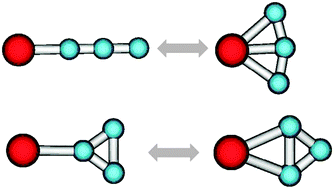
The molecular structures of third-row main group tricarbides C3X (X = K–Br) have been studied by quantum chemical methods. It is found that less electronegative elements (K, Ca, Ga, Ge) favor either fan or rhombic structures (resulting from side interactions with either linear or triangular C3 units), whereas the more electronegative elements (As, Se, Br) favor linear or three-membered ring structures (resulting from σ-type interactions with either linear or triangular C3 units). The predicted global minima are of fan type for C3K, rhombic for C3Ca, C3Ga, and C3Ge, linear for C3As and C3Se, and a three-membered ring for C3Br. In order to aid in their possible experimental identification the molecular geometries, vibrational frequencies,
- This article is part of the themed collection: Predicting new molecules by quantum chemical methods

 Please wait while we load your content...
Please wait while we load your content...