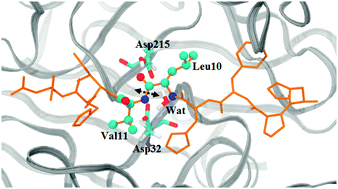
Hypertension is a chronic condition that affects nearly 25% of adults worldwide. As the Renin–Angiotensin–Aldosterone System is implicated in the control of blood pressure and body fluid homeostasis, its combined blockage is an attractive therapeutic strategy currently in use for the treatment of several cardiovascular conditions. We have performed QM/MM calculations to study the mouse renin catalytic mechanism in atomistic detail, using the N-terminal His6-Asn14 segment of angiotensinogen as substrate. The enzymatic reaction (hydrolysis of the peptidic bond between residues in the 10th and 11th positions) occurs through a general acid/base mechanism and, surprisingly, it is characterized by three mechanistic steps: it begins with the creation of a first very stable tetrahedral gem-diol intermediate, followed by protonation of the peptidic bond nitrogen, giving rise to a second intermediate. In a final step the peptidic bond is completely cleaved and both gem-diol hydroxyl protons are transferred to the catalytic dyad (Asp32 and Asp215). The final reaction products are two separate peptides with carboxylic acid and amine extremities. The activation energy for the formation of the gem-diol intermediate was calculated as 23.68 kcal mol−1, whereas for the other steps the values were 15.51 kcal mol−1 and 14.40 kcal mol−1, respectively. The rate limiting states were the reactants and the first transition state. The associated barrier (23.68 kcal mol−1) is close to the experimental values for the angiotensinogen substrate (19.6 kcal mol−1). We have also tested the influence of the density functional on the activation and reaction energies. All eight density functionals tested (B3LYP, B3LYP-D3, X3LYP, M06, B1B95, BMK, mPWB1K and B2PLYP) gave very similar results.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...