Abstract
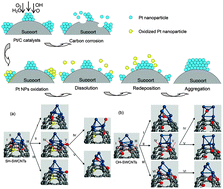
Using a combination of experiments and density functional theory (DFT) calculations, we explored the mechanisms of the stabilization effect of the thiolized (–SH) group on the Pt/SH-CNTs
- This article is part of the themed collection: Computational Catalysis and Materials for Energy Production, Storage and Utilization

 Please wait while we load your content...
Please wait while we load your content...