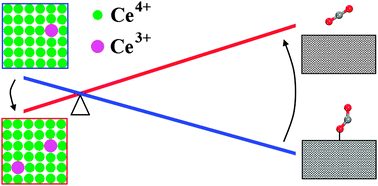
Density functional theory calculations corrected by on-site Coulomb interaction have been carried out to track down the lattice oxygen reactivity of CeO2(111) and (110) surfaces in direct oxidation of a single CO. The possible elementary steps in CO adsorption and subsequent reactions with lattice oxygen were systematically studied. From calculated energetics, we determined that the lattice oxygen of the (110) surface is more reactive than that of the (111) surface. By calculating the reaction pathways leading to different final products, we found that the formation of carbonate species is competitive to CO2 formation and desorption, and such an effect could be more significant at CeO2(110) compared to CeO2(111). More importantly, it has also been found that electron localization at the characteristic 4f orbital of Ce, directly determined by subtle structural relaxation, can give rise to a unique scenario of the overall reaction coordinates. These results may bring us one step ahead toward the comprehensive understanding of catalytic performance of CeO2-based materials.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...