Reactions of cisplatin and glycine in solution with constant pH: a computational study
Abstract
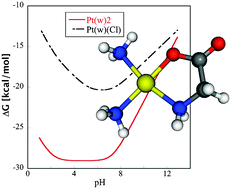
In this study interaction between glycine and cisplatin was explored. The structures were optimized in both gas phase and implicit water solution at the B3LYP/6-31G(p)/IEFPCM/UAKS computational level. Vibrational analysis confirms the corresponding character of all the complexes from examined reaction coordinates. The enthalpy and entropy contributions to Gibbs free energies were estimated from the vibrational frequencies and statistical canonical ensemble model. In the solution, several deprotonated forms were considered, and pKa values of the reactants and products were determined. The reaction Gibbs free energies and pKa values were used for determination of the transformed thermodynamic potential ΔG′ with pH as a natural variable.
- This article is part of the themed collection: Theoretical chemical physics of biological systems

 Please wait while we load your content...
Please wait while we load your content...