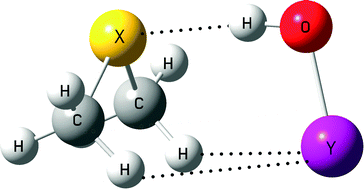
The complexes formed between dimethylchalcogens X(CH3)2 (X = S, Se, and Te) and hypohalous acids YOH (Y = F, Cl, Br, and I) have been studied at the MP2/aug'-cc-pVTZ computational level, five minima structures being located. Two of them correspond to hydrogen bonds (HB), another two to halogen bonds (XB) with the chalcogen acting as an electron donor, the last one showing a C–H⋯O contact. The most stable complexes of IOH and BrOH acids present halogen⋯chalcogen interactions with interaction energies, Ei, up to −49 kJ mol−1. In the case of the ClOH and FOH molecules, the hydrogen bonded complexes are more stable with interaction energies between −27 and −34 kJ mol−1. Linear correlations between the molecular electrostatic potential (MEP) stationary points at the van der Waals surface and the interaction energy have been found. The contribution of the different energy terms to the total interaction energy was analyzed by means of the DFT–SAPT theory finding that the electrostatic attractive term is dominant in the complexes with HB and XB, excepting a few cases in which the dispersion and induction terms become more important than the electrostatic one.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...