Photovoltaic characteristics and dye regeneration kinetics in D149-sensitized ZnO with varied dye loading and film thickness†
Abstract
Porous ZnO electrodes on fluorine-doped 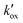 for D149–ZnO electrodes of systematically varied film thickness and
for D149–ZnO electrodes of systematically varied film thickness and 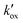 correlated directly with the adsorbed
correlated directly with the adsorbed 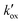 was found to be independent of the
was found to be independent of the
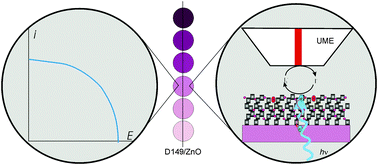

 Please wait while we load your content...
Please wait while we load your content...