Parahydrogen induced polarization in face of keto–enol tautomerism: proof of concept with hyperpolarized ethanol
Abstract
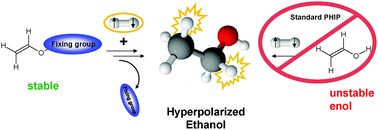
Hyperpolarization (HP) techniques are increasingly important in magnetic resonance imaging (MRI) and spectroscopy (MRS). HP methods have the potential to overcome the fundamentally low sensitivity of magnetic resonance (MR). A breakthrough of HP-MR in life sciences and medical applications is still limited by the small number of accessible, physiologically relevant substrates. Our study presents a new approach to extend PHIP to substrates that primarily cannot be hyperpolarized due to a steady intramolecular re-arrangement, the so-called keto–enol tautomerism. To overcome this obstacle we exploited the fact that instead of the instable enol form the corresponding stable ester can be used as a precursor molecule. This strategy now enables the hydrogenation which is required to apply the standard PHIP procedure. As the final step a hydrolysis is necessary to release the hyperpolarized target molecule. Using this new approach ethanol was successfully hyperpolarized for the first time. It may therefore be assumed that the outlined multi-step procedure can be used for other keto–enol tautomerized substances thereby opening the application of PHIP to a multitude of molecules relevant to analyzing metabolic pathways.

 Please wait while we load your content...
Please wait while we load your content...