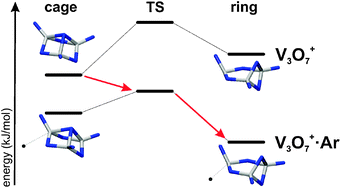
We present gas phase vibrational spectra of the trinuclear vanadium oxide cations V3O6+·He1–4, V3O7+·Ar0,1, and V3O8+·Ar0,2 between 350 and 1200 cm−1. Cluster structures are assigned based on a comparison of the experimental and simulated IR spectra. The latter are derived from B3LYP/TZVP calculations on energetically low-lying isomers identified in a rigorous search of the respective configurational space, using higher level calculations when necessary. V3O7+ has a cage-like structure of C3v symmetry. Removal or addition of an O-atom results in a substantial increase in the number of energetically low-lying structural isomers. V3O8+ also exhibits the cage motif, but with an O2 unit replacing one of the vanadyl oxygen atoms. A chain isomer is found to be most stable for V3O6+. The binding of the rare gas atoms to V3O6–8+ clusters is found to be strong, up to 55 kJ/mol for Ar, and markedly isomer-dependent, resulting in two interesting effects. First, for V3O7+·Ar and V3O8+·Ar an energetic reordering of the isomers compared to the bare ion is observed, making the ring motif the most stable one. Second, different isomers bind different number of rare gas atoms. We demonstrate how both effects can be exploited to isolate and assign the contributions from multiple isomers to the vibrational spectrum. The present results exemplify the structural variability of vanadium oxide clusters, in particular, the sensitivity of their structure on small perturbations in their environment.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...