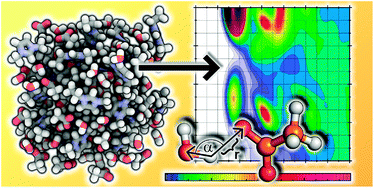
The ionic liquid 1-ethyl-3-methylimidazolium acetate [C2C1Im][OAc] shows a great potential to dissolve strongly hydrogen bonded materials, related with the presence of a strong hydrogen bond network in the pure liquid. A first step towards understanding the solvation process is characterising the hydrogen bonding ability of the ionic liquid. The description of hydrogen bonds in ionic liquids is a question under debate, given the complex nature of this media. The purpose of the present article is to rationalise not only the existence of hydrogen bonds in ionic liquids, but also to analyse their influence on the structure of the pure liquid and how the presence of water, an impurity inherent to ionic liquids, affects this type of interaction. We perform an extensive study using ab initio molecular dynamics on the structure of mixtures of the ionic liquid 1-ethyl-3-methylimidazolium acetate with water, at different water contents. Hydrogen bonds are present in the pure liquid, and the presence of water modifies and largely disturbs the hydrogen bond network of the ionic liquid, and also affects the formation of other impurities (carbenes) and the dipole moment of the ions. The use of ab initio molecular dynamics is the recommended tool to explore hydrogen bonding in ionic liquids, as an explicit electronic structure calculation is combined with the study of the condensed phase.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...