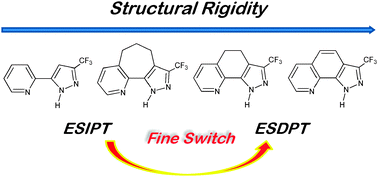
A series of 2-pyridyl-pyrazole derivatives 1–4 possessing five-membered ring hydrogen bonding configuration are synthesized, the structural flexibility of which is strategically tuned to be in the order of 1 > 2 > 3 > 4. This system then serves as an ideal chemical model to investigate the correlation between excited-state intramolecular proton transfer (ESIPT) reaction and molecular skeleton motion associated with hydrogen bonds. The resulting luminescence data reveal that the rate of ESIPT decreases upon increasing the structural constraint. At sufficiently low concentration where negligible dimerization is observed, ESIPT takes place in 1 and 2 but is prohibited in 3 and 4, for which high geometry constraint is imposed. The results imply that certain structural bending motions associated with hydrogen bonding angle/distance play a key role in ESIPT. This trend is also well supported by the DFT computational approach, in which the barrier associated with ESIPT is in the order of 1 < 2 < 3 < 4. Upon increasing the concentration in cyclohexane, except for 2, the rest of the title compounds undergo ground-state dimerization, from which the double proton transfer takes place in the excited state, resulting in a relatively blue shifted dimeric tautomer emission (cf. the monomer tautomer emission). The lack of dimerization in 2 is rationalized by substantial energy required to adjust the angle of hydrogen bond via twisting the propylene bridge prior to dimerization.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...