Mechanism investigation of ketone hydrogenation catalyzed by ruthenium bifunctional catalysts: insights from a DFT study†
Abstract
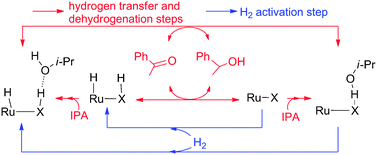
In this paper, the mechanism of ketone hydrogenation catalyzed by five Ru bifunctional catalysts with different structural frameworks was studied in detail using density functional theory (DFT). This mechanism contains hydrogen transfer, dehydrogenation of alcohol, and dihydrogen activation fundamental reactions. The involvement of alcohol is also discussed and found with different activities in hydrogen transfer, dehydrogenation and dihydrogen activation steps in five systems. Our calculated results indicate that the weak Ru–H bond, stronger basicity of hydride and stronger X–H acidity will decrease the barrier of the HT step, and that the polar micro-environment of dihydrogen coordinating with Ru catalysts and short hydrogen transfer distance would be able to facilitate the heterolytic splitting of dihydrogen in the dihydrogen activation step.

 Please wait while we load your content...
Please wait while we load your content...