Kinetics of the iodate reduction by hydrogen peroxide and relation with the Briggs–Rauscher and Bray–Liebhafsky oscillating reactions†
Abstract
The iodate reduction by hydrogen peroxide in acidic solutions is part of the Bray–Liebhafsky and Briggs–Rauscher oscillating reactions. At low hydrogen peroxide concentrations, typical of the Bray–Liebhafsky reaction, its rate law is 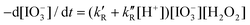 with
with 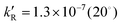 , 7.8 × 10−7 (39°), 1.4 × 10−5 M−1 s−1 (60°) and
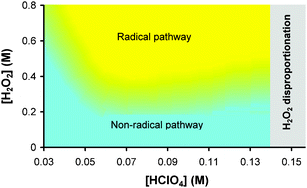
, 7.8 × 10−7 (39°), 1.4 × 10−5 M−1 s−1 (60°) and 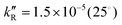 , 6.2 × 10−5 (39°), 6.3 × 10−4 M−2 s−1 (60°). It is explained by a non-radical mechanism. At high hydrogen peroxide concentrations, typical of the Briggs–Rauscher reaction, a new reaction pathway appears with a rate more than proportional to [H2O2]2 and nearly independent of [IO3−] > 0.01 M. This pathway is inhibited by scavengers of free radicals. We suggest that it has a radical mechanism starting with IOH + H2O2 ⇌ IOOH + H2O and IOOH+H2O2 → IO˙ + H2O+HOO˙.
, 6.2 × 10−5 (39°), 6.3 × 10−4 M−2 s−1 (60°). It is explained by a non-radical mechanism. At high hydrogen peroxide concentrations, typical of the Briggs–Rauscher reaction, a new reaction pathway appears with a rate more than proportional to [H2O2]2 and nearly independent of [IO3−] > 0.01 M. This pathway is inhibited by scavengers of free radicals. We suggest that it has a radical mechanism starting with IOH + H2O2 ⇌ IOOH + H2O and IOOH+H2O2 → IO˙ + H2O+HOO˙.

 Please wait while we load your content...
Please wait while we load your content...