Kinetic analysis of the thermal isomerisation pathways in an asymmetric double azobenzene switch†
Abstract
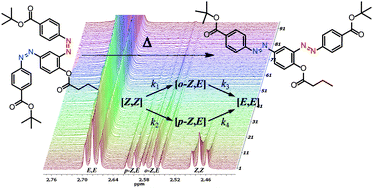
Here we report a photochemical and kinetic study of the thermal relaxation reaction of a double azobenzene system, in which two azobenzene photochromic units are connected via a phenyl ring. Upon UV irradiation, three thermally unstable isomers are formed. Kinetic studies using arrayed 1H-NMR spectroscopy revealed four distinct barriers for the thermal reversion to the stable isomer. The double isomerised Z,Z-2 can revert thermally to the E,E-2 isomer via either of two isomerisation pathways. The thermal Z to E isomerisations are not significantly affected by the state of the neighbouring azo-switching unit in the meta position. These findings are supported by quantum chemical calculations on the thermal Z to E isomerisation.

 Please wait while we load your content...
Please wait while we load your content...