Light harvestingzinc naphthalocyanine–perylenediimide supramolecular dyads: long-lived charge-separated states in nonpolar media†
Abstract
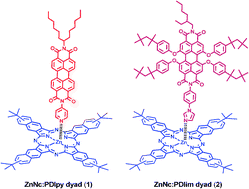
Photoinduced electron-transfer dynamics of self-assembled donor–acceptor dyads formed by axial coordination of
* Corresponding authors
a
Department of Material and Life Science, Graduate School of Engineering, Osaka University, ALCA, Japan Science and Technology Agency (JST), Suita, Japan
E-mail:
fukuzumi@chem.eng.osaka-u.ac.jp, melkhouly@chem.eng.osaka-u.ac.jp
Fax: +81-6-6879-7370
Tel: +81-6-6879-7369
b Department of Chemistry, Faculty of Science, Kafr ElSheikh, Kafr ElSheikh University, Egypt
c
División de Química Orgánica, Instituto de Bioingeniería, Universidad Miguel Hernández de Elche, Elche, Spain
E-mail:
asastre@umh.es, fdofdez@umh.es
d Department of Bioinspired Science, Ewha Woman's University, Seoul, Korea
Photoinduced electron-transfer dynamics of self-assembled donor–acceptor dyads formed by axial coordination of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
M. E. El-Khouly, A. M. Gutiérrez, Á. Sastre-Santos, F. Fernández-Lázaro and S. Fukuzumi, Phys. Chem. Chem. Phys., 2012, 14, 3612 DOI: 10.1039/C2CP23285E
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
