Modification of the secondary structure of angiotensin II by substitution of hydrogen with Cs cations: an experimental and theoretical study
Abstract
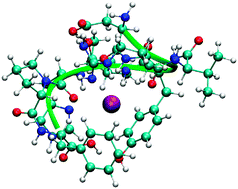
MALDI mass spectrometry in combination with post-source decay (PSD) analysis is a fast and easy to apply method for peptide sequencing. In this study, the PSD technique was used to investigate the influence of the adaption of one, two, and three caesium cations to angiotensin II in the gas phase. The PSD spectra of caesium-aggregated angiotensin II show far less fragmentation in comparison to the protonated one. In the case of singly (doubly) Cs+ substituted angiotensin II, the PSD mass spectrum shows only fragments with one (two) Cs cation(s). These results are interpreted in terms of additional interactions of the caesium cation(s) with the peptide. In order to investigate this suggestion, the molecular structures were calculated with semi-empirical molecular dynamic (MD) simulations and further optimized at the quantum chemical level (BP86, SVP) of theory. On the one hand, secondary structures of Cs+ substituted angiotensin II are more compact than the structure of protonated angiotensin II, indicating electrostatic interactions of the Cs cations and the heterocyclic structures. Moreover, oxyphilic interactions of the cations with the oxygen atoms of the peptide backbone also contribute as further van-der-Waals interactions of the Cs+ substituted angiotensin II. These interactions are able to explain its higher stability due to reduced dissociation in comparison to the protonated angiotensin II. On the other hand, most MD simulations of doubly and triply Cs+ substituted angiotensin II show a formation of a [2 Cs] cluster, surrounded by the peptide molecule. The formation of this cluster would explain the lack of singly Cs+ substituted fragments in the PSD mass spectrum of doubly Cs+ substituted angiotensin II.
- This article is part of the themed collection: Structure and reactivity of small particles: from clusters to aerosols

 Please wait while we load your content...
Please wait while we load your content...