Chemical and electrochemical insertion of Li into the spinel structure of CuCr2Se4: ex situ and in situ observations by X-ray diffraction and scanning electron microscopy
Abstract
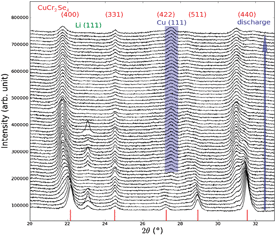
The chemical and electrochemical insertion of lithium into the spinel structure of CuCr2Se4 was studied and the chemical reaction pathway was followed by ex situ

 Please wait while we load your content...
Please wait while we load your content...