Hydrogen peroxide triggered morphological evolution of barium sulfate crystals under basic conditions†
Abstract
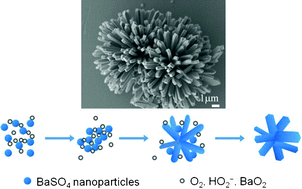
Inorganic small molecule H2O2 was utilized as an additive to control the crystallization of BaSO4. The evolution from simple flake-like to pillow morphologies shaped with dominant (hk0) planes and (001) plane was observed by just increasing the pH value and the H2O2 concentration in the reaction solution. Accompanying the change, the hexagonal (001) plane becomes the main face with the side faces transforming into the (210) and (100) faces. The inhibition on the [001] direction results in the appearance of a (001) face and the faster growth in the [010] direction influences the shape of the (001) plane to be hexagonal, elongated along the [010] direction. The crystalline morphologies of urchin-like BaSO4 at different mineralization times were investigated by SEM and a four-step evolution mechanism was proposed. Selective adsorption of HO2− originated from the decomposition of H2O2 over the BaSO4 particles, following gradual O2 evolution, and the formation of BaO2 were considered to be the key factors in the structural evolution. The structural units of urchin-like BaSO4 were strips with hexagonal basal faces, which could be seen as defect-free one-dimensional single crystals. The continuous variation of the morphology captured in this work may provide a deeper understanding of the formation mechanism of one-dimensional barium sulfate fibers. In addition, the results indicate that H2O2 may act as an additive for the crystallization of minerals. Because the final decomposition products are H2O and O2, H2O2 is especially suitable for directing the crystallization demanding high purity of the products.

 Please wait while we load your content...
Please wait while we load your content...