Chirality determination from X-ray powder data—diastereomeric co-crystals of mandelic acid and proline amide†
Abstract
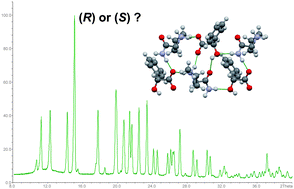
Diastereomeric co-crystals of the title compounds display sufficiently different powder diffractograms in order to determine the absolute configuration of one of the two crystal formers. Selective formation of the more stable diastereomer is observed if racemic conglomerates or

 Please wait while we load your content...
Please wait while we load your content...