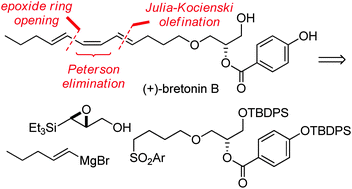
Total synthesis of (+)-bretonin B: access to the (E,Z,E)-triene core by a late-stage Peterson elimination of a convergently assembled silyl ether†
Abstract
The title compound was synthesised in a concise route (nine linear steps, 31% overall yield) employing an α-silyl epoxide ring opening, a Julia–Kocienski olefination and a late-stage Peterson elimination as key steps.

 Please wait while we load your content...
Please wait while we load your content...