Unusual para-substituent effects on the intramolecular hydrogen-bond in hydrazone-based switches†
Abstract
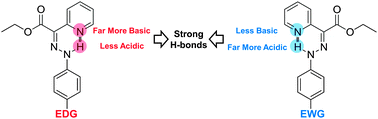
A “V”-shaped Hammett plot shows that resonance-assisted hydrogen bonding does not dictate the strength of the intramolecular hydrogen bond in the E isomers of
- This article is part of the themed collection: Aromaticity

 Please wait while we load your content...
Please wait while we load your content...