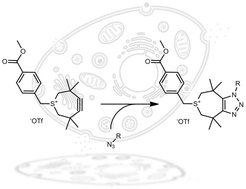
A new derivative of the strained 3,3,6,6-tetramethylthiacycloheptyne (TMTH) bearing a functional handle is reported. Following an optimized synthesis, the handle was introduced by mild alkylation of the sulphur atom. The resulting functionalized strained 4,5-didehydro-3,3,6,6-tetramethyl-2,3,6,7-tetrahydrothiepinium (TMTI) proved to be stable and underwent extremely fast [3+2] cycloaddition reaction with benzyl azide in both organic and aqueous solvents. The reaction was equally efficient in cell lysate and serum and therefore opens interesting prospects for chemical-biology applications.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...