An efficient synthesis of 2-bromo(chloro)-3-selenyl(sulfenyl)indoles via tandem reactions of 2-(gem-dibromo(chloro)vinyl)anilines with diselenides(disulfides)†
Abstract
A novel and efficient synthesis of 2-bromo(chloro)-3-selenyl(sulfenyl)indoles through tandem reactions of 2-(gem-dibromo(chloro)vinyl)-N-methylsulfonylanilines with
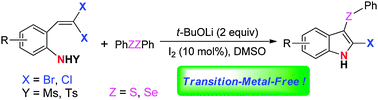

 Please wait while we load your content...
Please wait while we load your content...