Abstract
Starting from methyl 3,5-di-O-benzyl-2-keto-α-D-ribofuranoside, a convergent, six-step synthesis is developed to give efficiently all four 2′-C-α-aminomethyl-2′-deoxynucleosides (U, C, A, G) in 38%, 42%, 12%, 12% yield, respectively. Convergence is achieved by the glycosylation of persilylated
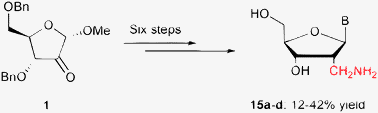
- This article is part of the themed collection: Nucleic acids: new life, new materials

 Please wait while we load your content...
Please wait while we load your content...