Synthesis of strained cyclic peptides via an aza-Michael–acyl-transfer reaction cascade†
Abstract
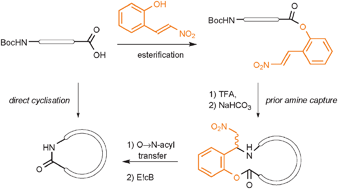
A new method is presented to prepare strained lactams. Esterification of the C-terminus of a dipeptide with β-nitrostyrene or quinoline-type auxiliaries is followed by lactam formation by an intramolecular aza-Michael–acyl-transfer reaction cascade. Ultimately, the cyclic tetrapeptide cyclo[Phe-Tyr-Ala-Gly] has been prepared.

 Please wait while we load your content...
Please wait while we load your content...