Design of chiral sulfoxide–Schiff base hybrids and their application in Cu-catalyzed asymmetric Henry reactions†
Abstract
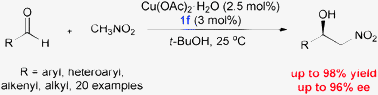
A new class of chiral sulfoxide–Schiff base ligands has been developed by the rational combination of two privileged chiral backbones. These sulfoxide–Schiff base ligands were found to be highly efficient for Cu-catalyzed asymmetric Henry reactions (up to 98% yield and 96% ee).

 Please wait while we load your content...
Please wait while we load your content...