Enantioselective Friedel–Crafts reactions between phenols and N-tosylaldimines catalyzed by a leucine-derived bifunctional catalyst†
Abstract
Enantioselective Friedel–Crafts reactions between
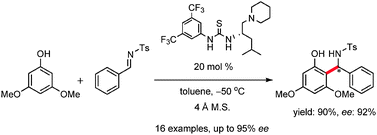
- This article is part of the themed collection: Chirality

 Please wait while we load your content...
Please wait while we load your content...