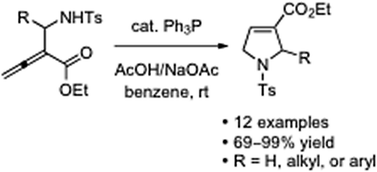
Phosphine-catalyzed intramolecular γ-umpolung addition of α-aminoalkylallenic esters: facile synthesis of 3-carbethoxy-2-alkyl-3-pyrrolines†‡
Abstract
An array of N-tosylated α-aminoalkylallenic
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...