Hydrogenation effects in metalloporphycenes: synthesis and redox behavior of Ni(ii)–tetra(n-propyl)dihydroporphycene†‡
Abstract
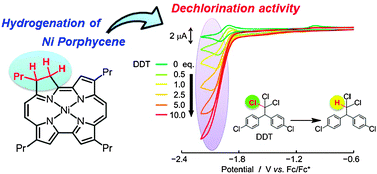
Hydrogenated tetrapropylporphycenes, 2,3-dihydro-2,7,12,17-tetrapropylporphycene 1 and its NiII complex 2, have been prepared and the hydrogenation effects on their electronic structure characterized. A one-electron
- This article is part of the themed collection: Porphyrins & Phthalocyanines

 Please wait while we load your content...
Please wait while we load your content...