Redox tuning of two biological copper centers through non-covalent interactions: same trend but different magnitude†
Abstract
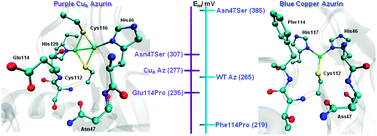
The same non-covalent interactions previously found to affect the redox potential (Em) of the mononuclear T1 Cu protein azurin (Az) are shown to also fine-tune the Em of the dinuclear CuA center in the same Az protein scaffold. The effects of these mutations are in the same direction but with smaller magnitude in the CuA site, due to dissipation of the effects by the dinuclear CuA center.

 Please wait while we load your content...
Please wait while we load your content...