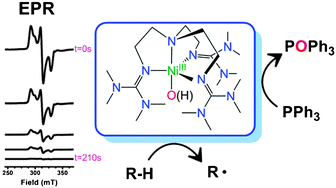
Spectroscopic capture and reactivity of S = 1/2 nickel(iii)–oxygen intermediates in the reaction of a NiII-salt with mCPBA†‡
Abstract
Ni(III)-intermediates are trapped by
- This article is part of the themed collection: Porphyrins & Phthalocyanines

 Please wait while we load your content...
Please wait while we load your content...