Palladium-catalyzed deamidative arylation of azoles with arylamides through a tandem decarbonylation–C–H functionalization†
Abstract
A highly chemo-, regio-selective, and efficient
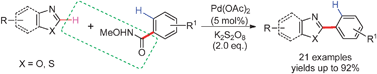
- This article is part of the themed collection: C-H Functionalization

 Please wait while we load your content...
Please wait while we load your content...