Partially saturated fluorinated heterocycles: diastereo- and enantioselective synthesis of β-trifluoromethyl-pyrroline carboxylates†‡
Abstract
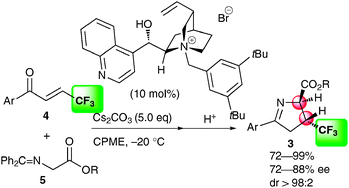
The first asymmetric synthesis of β-trifluoromethylated
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...