Differentiation of diastereotopic bromine atoms in SN2 reactions of gem-dibromides†
Abstract
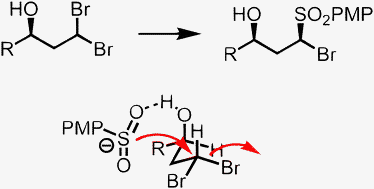
A novel directed SN2 reaction of conformationally biased gem-dibromides and an arenesulfinate anion is described. The reaction results in the diastereoselective formation of α-bromosulfones. The selectivity originates from pre-coordination of the nucleophile to a free hydroxyl group in the γ-position.

 Please wait while we load your content...
Please wait while we load your content...