Hexafluorobenzene: a powerful solvent for a noncovalent stereoselective organocatalytic Michael addition reaction†‡
Abstract
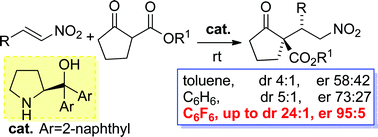
A dramatic enhancement of the diastereo- and enantioselectivity in the nitro-Michael addition reaction organocatalysed by a commercially available α,α-L-diaryl prolinol was disclosed when performing the reaction in unconventional
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...