Discovery of 2-aminobuta-1,3-enynes in asymmetric organocascade catalysis: construction of drug-like spirocyclic cyclohexanes having five to six contiguous stereocenters†‡
Abstract
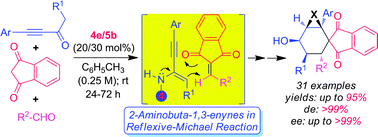
We present herein for the first time the asymmetric synthesis of
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...