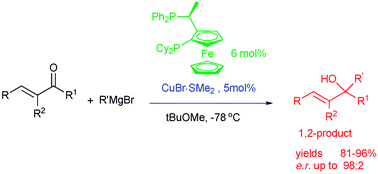
Copper(i) catalyzed asymmetric 1,2-addition of Grignard reagents to α-methyl substituted α,β-unsaturated ketones†‡
Abstract
The first catalytic enantioselective
- This article is part of the themed collection: Emerging Investigators 2012

 Please wait while we load your content...
Please wait while we load your content...